Anodic oxidation
Anodic oxides
The growth of anodic oxides in aqueous solutions is a process with implications in electrocatalysis and corrosion. In the case where the solvent is methanol instead of water, the anodic oxidation of copper results in two types of surface species as well as electrodissolution. The surface species created during the anodic oxidation of copper in a naturally aerated methanolic solution may be similar to the ones observed during the anodic oxidation of copper in mild basic aqueous solutions. Dissolved oxygen seems to be responsible for the anodic oxidation of copper to its oxides whereas the oxidation of copper in deaerated methanolic solutions results only in metal dissolution.
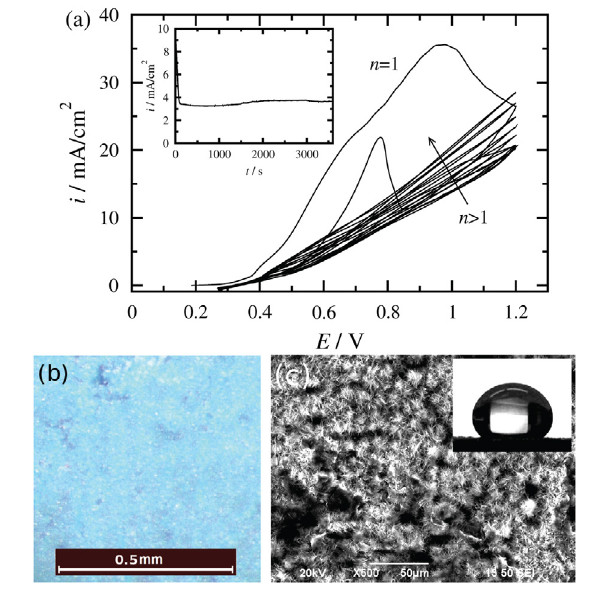
Hydrophobic films
Alcoholic solutions of saturated fatty acids, together with appropriate inorganic cations are often used for the fabrication of hydrophobic layers by a one-step anodic process. Hydrophobic surfaces are characterized by static water contact angle above 90 degrees while superhydrophobic surfaces have a contact angle of above 150 degrees. The hydrophobic property is a result of the combination of specific surface morphology and chemical composition of the layer. In the case of anodic fabrication, the resulting layer is an organo-metallic salt composed by the organic anion and the inorganic cation provided from the electrode. The fatty acids commonly employed are stearic and myristic acid.
Chlorine production
The electrochemical production of chlorine, by electrolysis of brine, is one of the major processes in the chemical industry and one of the earliest commercial exploitations of an electrochemical reaction on a large scale. Electrochemical disinfection can be defined as the eradication of microorganisms using an electric current passed through the water by means of suitable electrodes. A typical reactor, implementing electrolysis for chlorine production contains a configuration of feeder and bipolar electrodes and can be used for ballast water treatment.
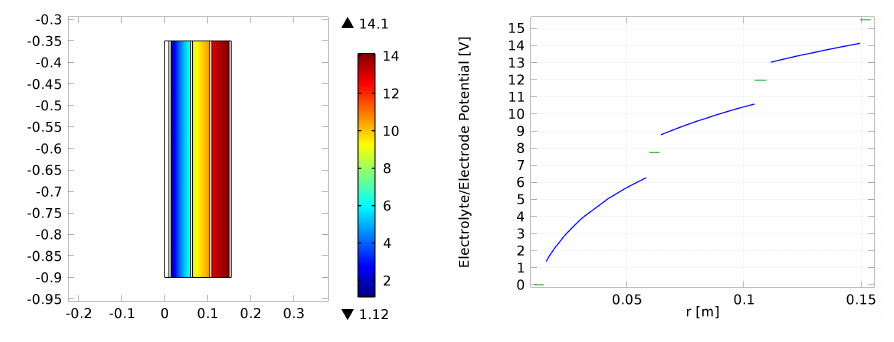
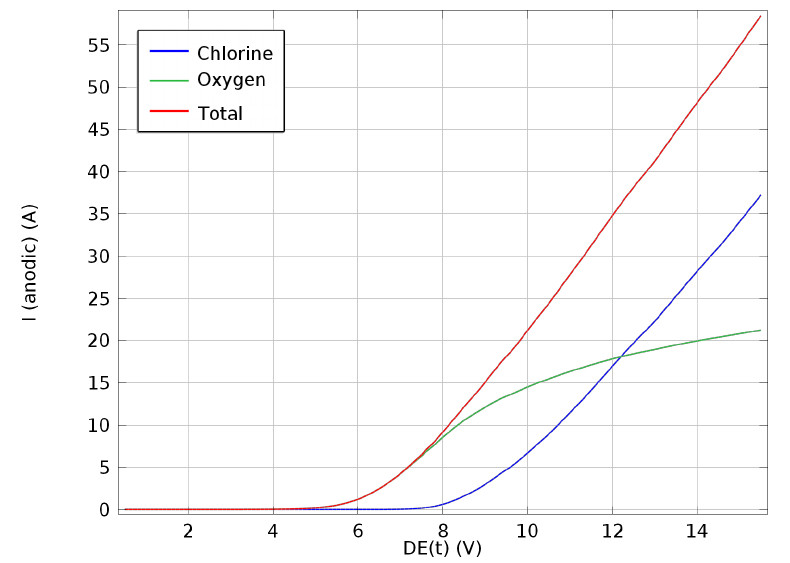
Super-capacitors
Conducting polymers are candidate materials for the construction of supercapacitor electrodes. Conducting polymers such as polyaniline are fabricated by electrochemical or chemical polymerization on different conducting substrates such as platinum, mixed mode oxides, stainless steel or nickel. The performance of such plain or modified electrodes is evaluated by charge - discharge curves, cyclic voltammetry and electrochemical impedance spectroscopy.
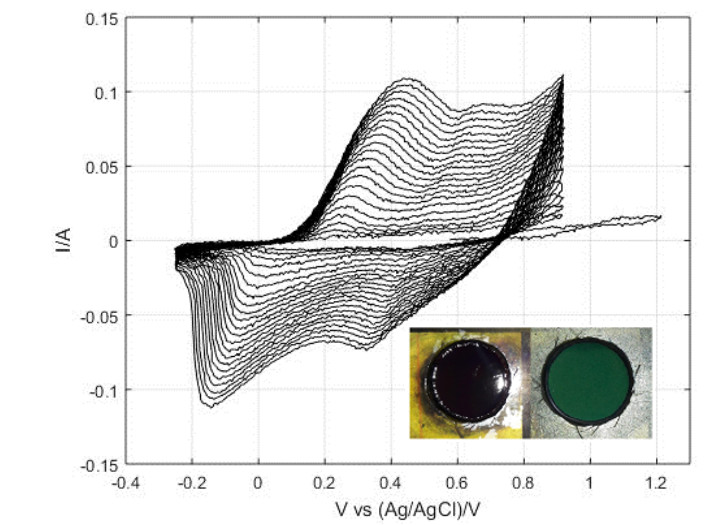
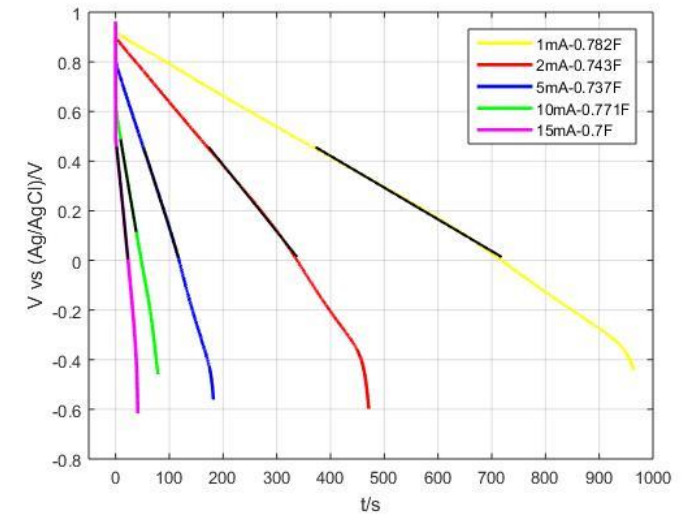
Cathodic deposition
Metals and composites
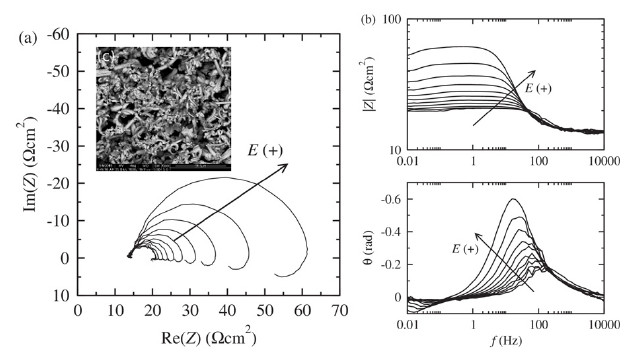
Composite coatings are obtained by electrolytic co-deposition of hybrid multi wall carbon nanotubes and alumina and nickel The mechanism of electro- chemical co-deposition of Ni and hybrid MWCNTs is investigated by linear sweep voltammetry, electrochemical impedance spec- troscopy and quartz crystal microbalance measurements in order to elucidate some aspects of the mechanism of electrochemical co-deposition of Ni with hybrid MWCNT particles. Experimental findings are interpreted by means of kinetic models for nickel deposition from acidic baths.
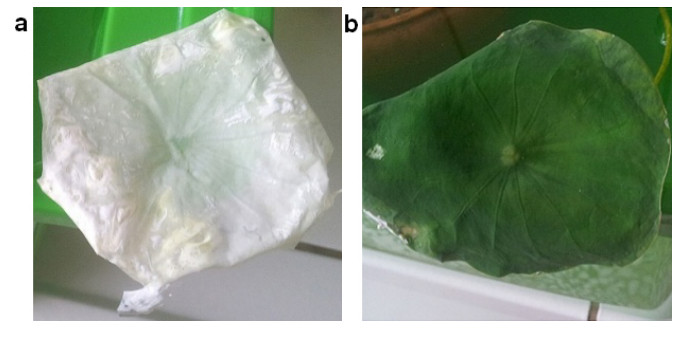
Hydrophobic films
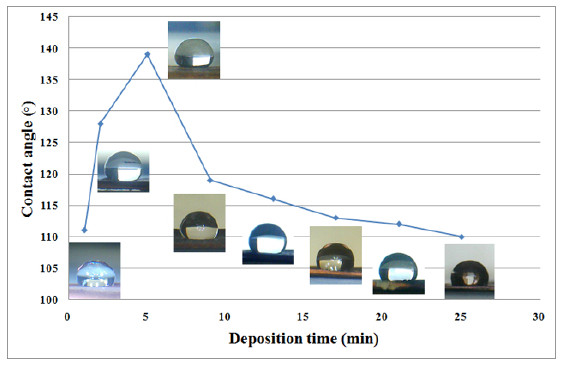
By mimicking nature and combining a micro-nano structured surface with a low energy material deposited on it, an increase of the static contact angle can be achieved, repelling water molecules more efficiently and leading to an improved super-hydrophobic behavior. Fabrication of biomimetic surfaces for their use as deposition substrates is possible, aiming to take advantage of the micro-nano structure of various natural hydrophobic surfaces, such as plant leaves. Different biotextures can be replicated for this purpose, and different coating materials can be used. The properties of each biotexture and coating depent on deposition parameters and affect the contact angle and adhesion.
Rare earths
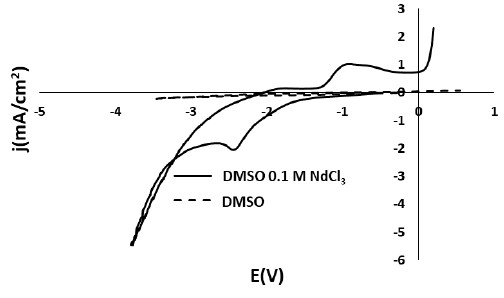
Electrodeposition of rare earths can be achieved, to some extent, from organic solvents or ionic liquids. Cyclic voltammetry is implemented in order determine the reactions taking place as well as the stability of the solvent. Optical and spectroscopic techniques offer means for the evaluation of the deposided rare earth.
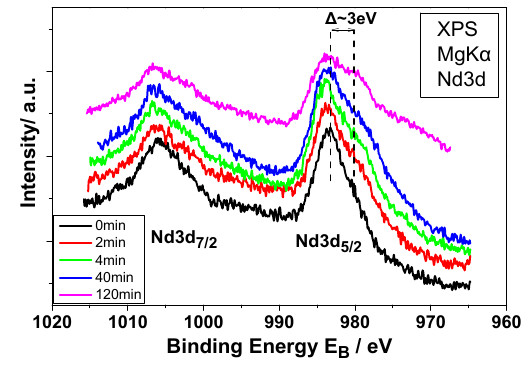
Bioelectrochemistry
Enzyme kinetics
Redox enzymes are a very important class of enzymes that can also be studied with electrochemical methods. The most prominent studies are through the use of mediators and the use of cyclic voltammetry, but there has been an effort for the study with direct electron transfer as well. The extraction of the kinetic information can be obtained with the use of analytical solutions or fitting of experimental data.
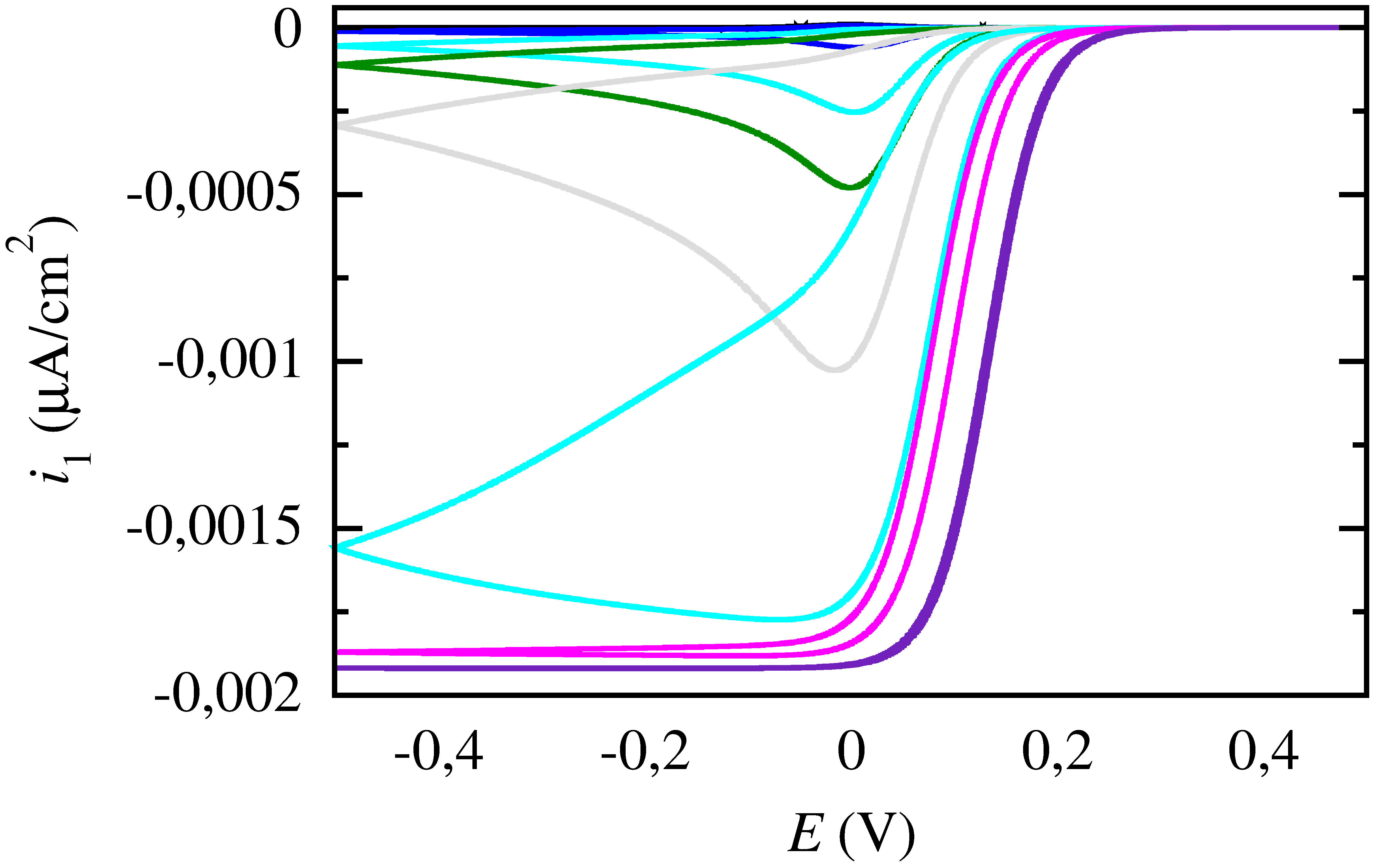
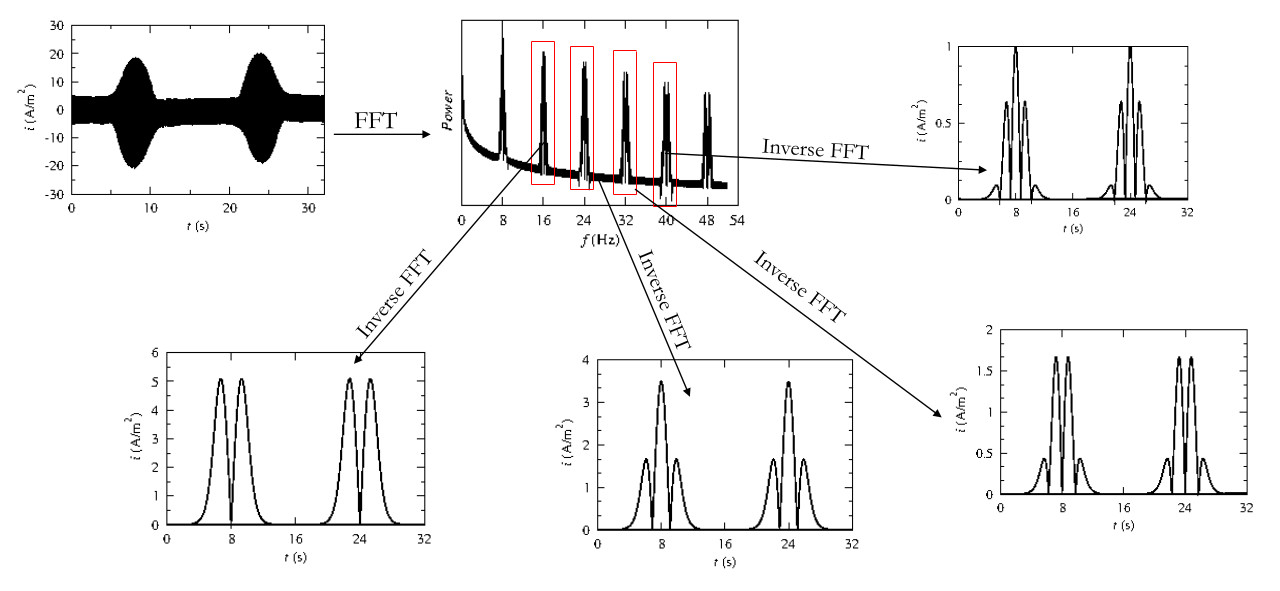
Techniques
The classic method for electrochemical studies of substasces is cyclic voltammetry as it is the most widely used. However, when it comes to the study of enzymes or molecules at low concenatrions limitations such as capacitance currents are faced. LA-FTacV (large amplitude Fourier transform alternate current voltammetry), an alteration to the classic cyclic voltammetry, involving a sinusoidal pertubation of large amplitude (above 80 mV), which eliminates the capapcitance and leads to pure faradaic currents is a promising technique that could contribute to the study of such molecules. It also reveals more information on the kinetic mechanisms of redox reactions in comparison to cyclic voltammetry and amplifies the electrochemical signal
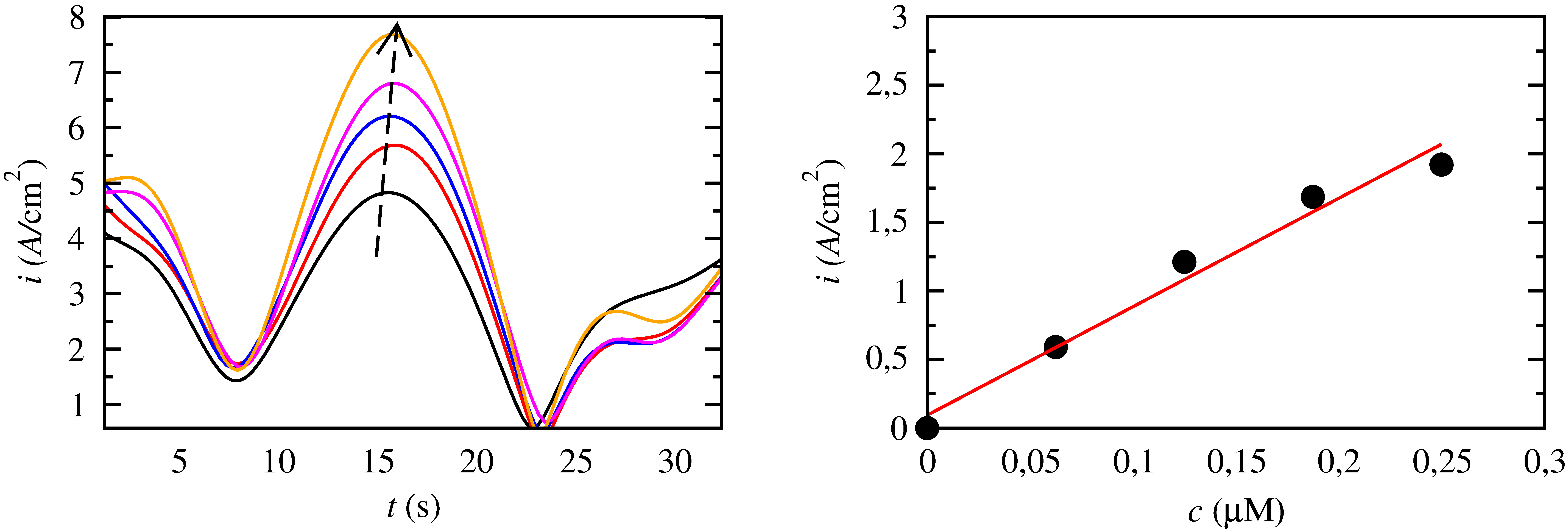
Bio-sensors
Redox enzymes immobilized on electrode surfaces are widely used for the detection of small concnetrations of different substanses. Second generation biosensors, which need the use of a mediated system, are mostly predominant in the literature but in the past few years, third generation biosensors which rely on the direct electron transfer of an enzyme and an electrode surface have emerged. Enzymes such as peroxidases and laccases can be employed for the above purposes detecting different substances of high affinity with these enzymes.
Non-linear electrochemistry
Oscillatory electrode reactions
A variety of electrochemical reactions exhibit oscillatory behaviors, such as the electrodissolution of iron, nickel, copper, the catalytic oxidation of small organic molecules etc. The instabilities leading to oscillary behavior are analyzed by applying concepts of bifuraction theory and time series analysis as well as appropriate electrochemical techniques.
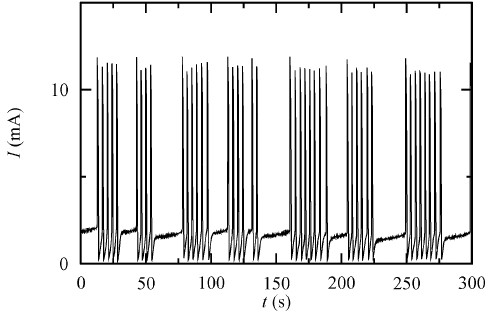
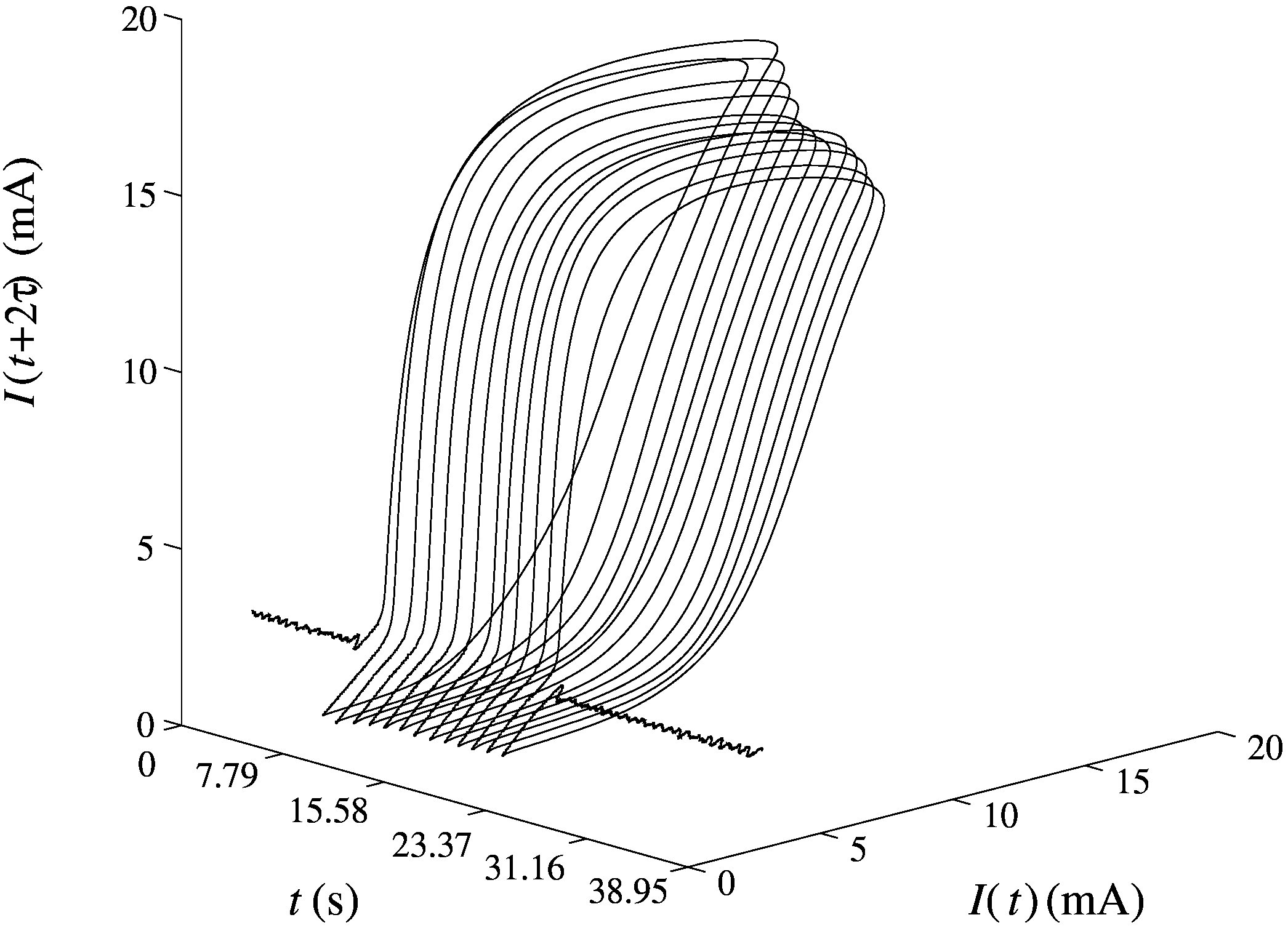
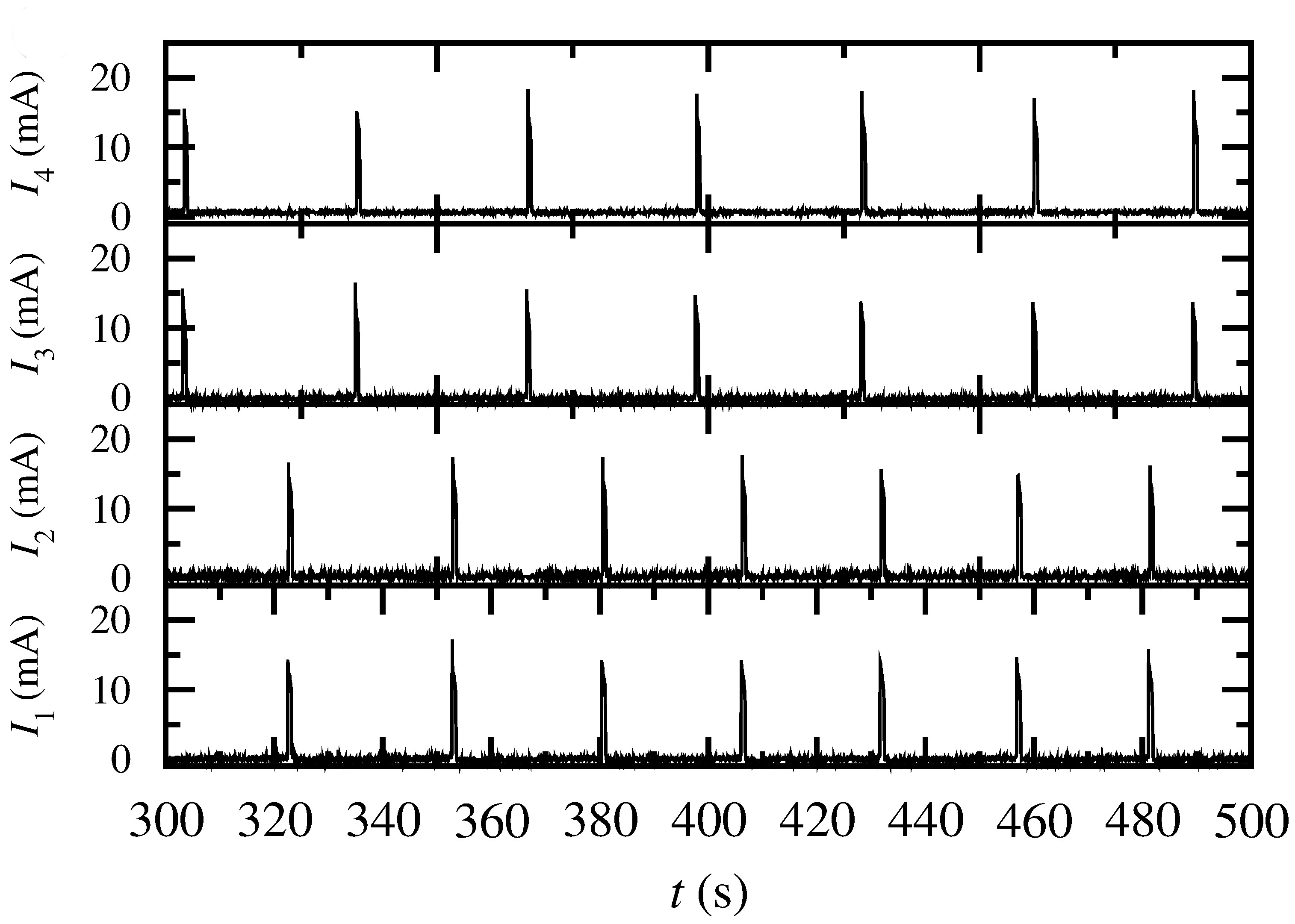
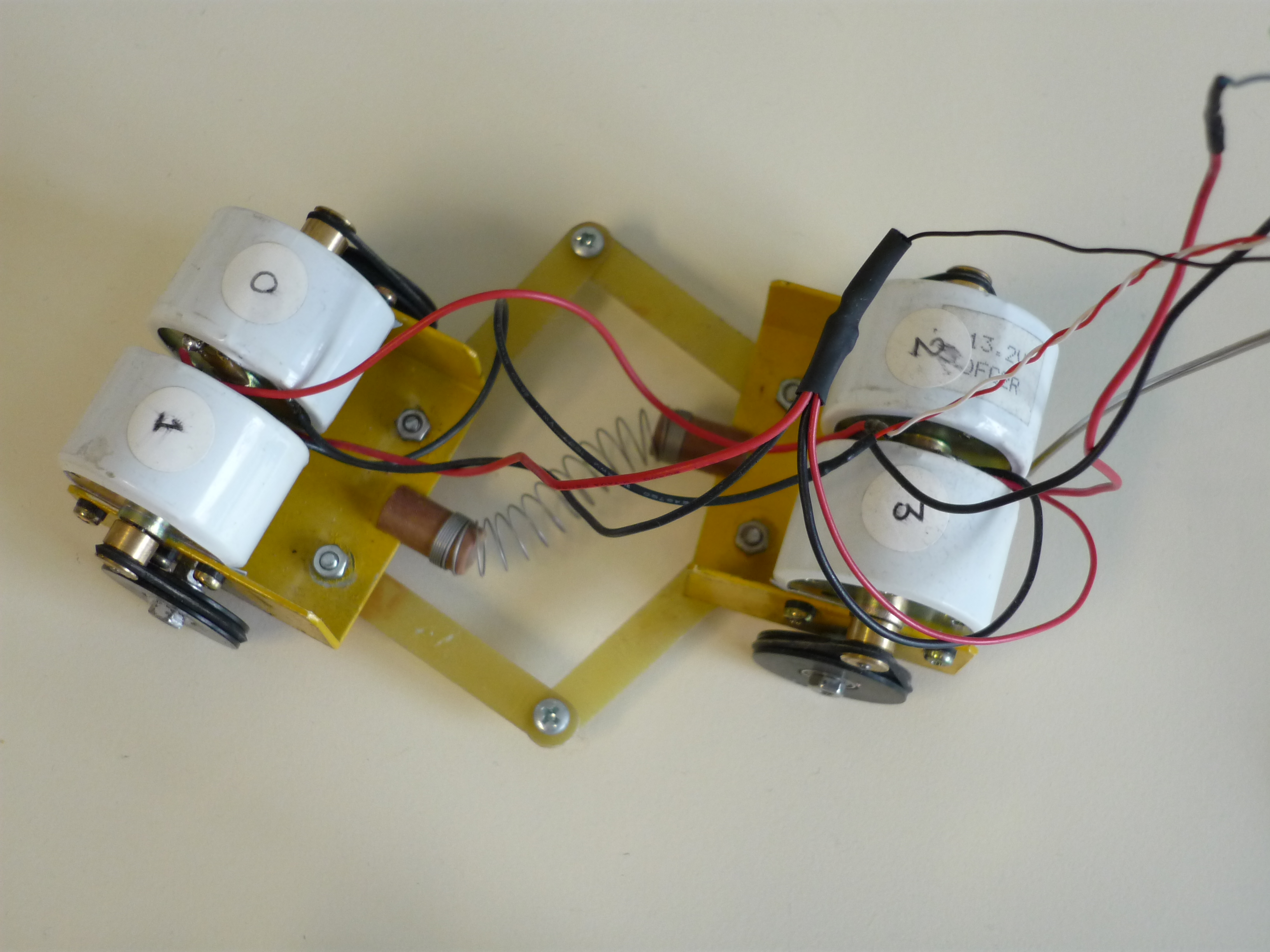
Electrochemical networks
A variety of electrochemical systems are capable of producing high amplitude electric pulses, either autonomously or under perturbation, and behave as oscillators or excitators. Moreover, these systems have the ability to communicate through the electrolytic solution while in proximity and thus form networks capable of various types of synchronization. Electrochemical oscillatory networks can function as central patterns generators (CPGs) and control the locomotion of mechanical devices.
Electrochemical resonance
Electrochemical devices can be implemented as pseudo transistors, elec- trical switches, diodes and rectifiers. It has been claimed also that, among other uses in electronics, electrochemical capacitors can be utilized for low-frequency electrical filtering. Indeed, the electrochemical interface can act as a band-pass filter under specific conditions. This behavior is based on the combination of the dynamic and electric properties of the electrochemical interface enabling the system to resonate, that is, to exhibit a maximum of the admittance at a specific resonance frequency.
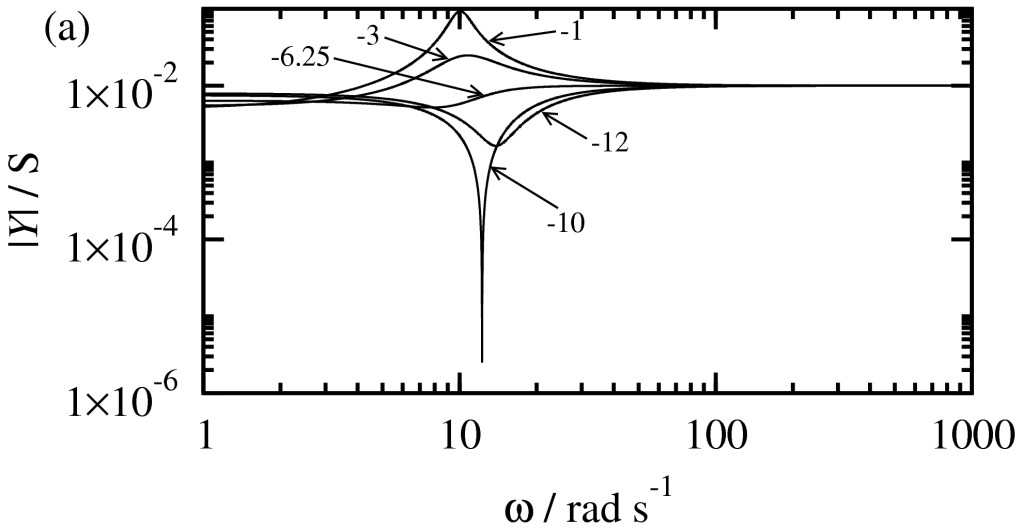
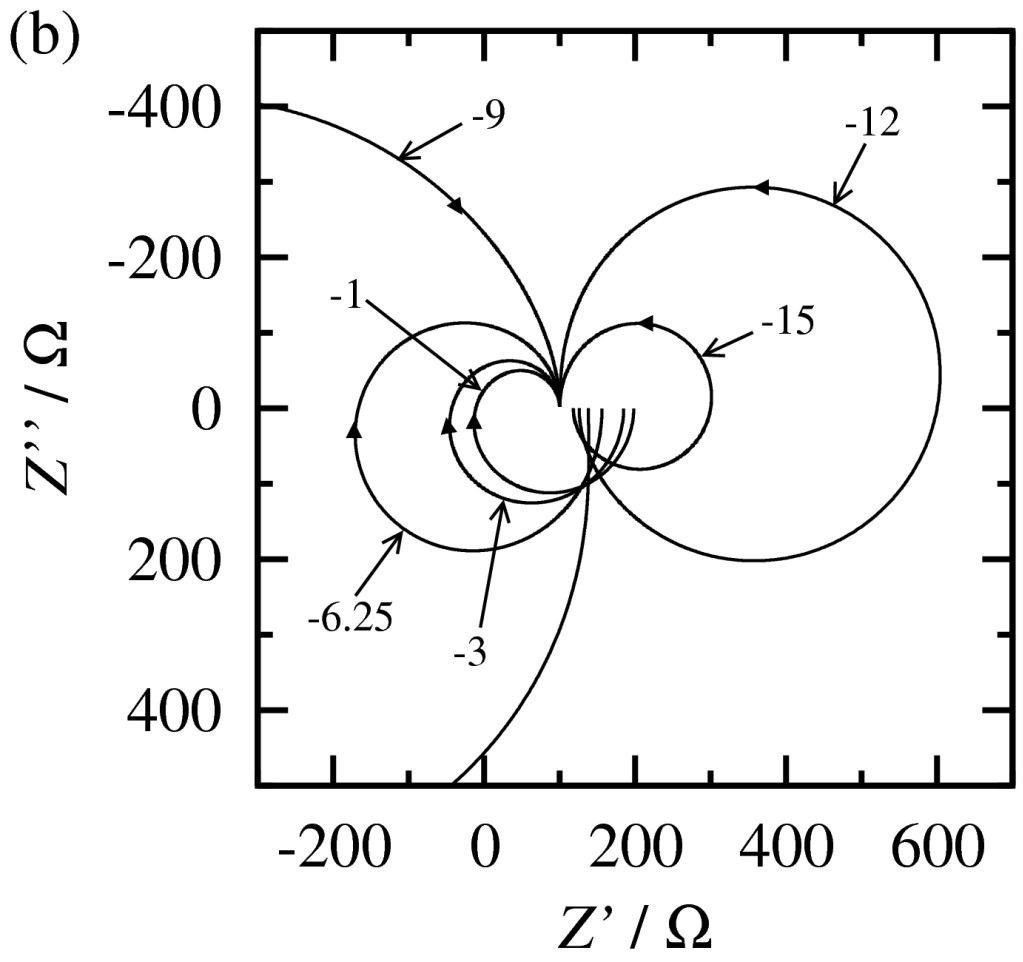
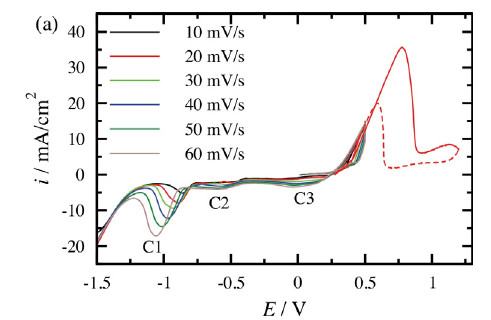
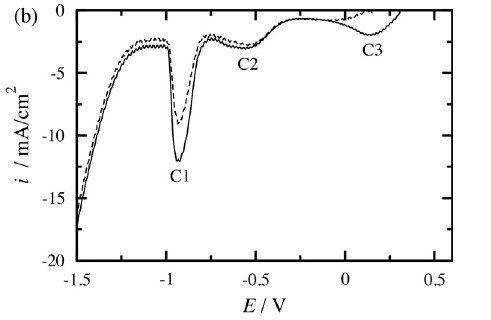
Corrosion of metals
Corrosion and inhibition
Corrosion inhibitors provide means for corrosion protection. These compounds are often incorporated in an organic coating and they protect the metal by covering the surface by passive layers or complexes. Several different systems have been investigated, i.e. combination of metal, coating and inhibitor, mainly by electrochemical impedance spectroscopy.
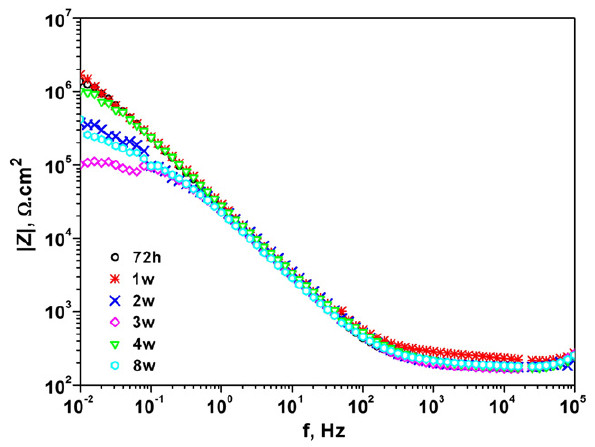
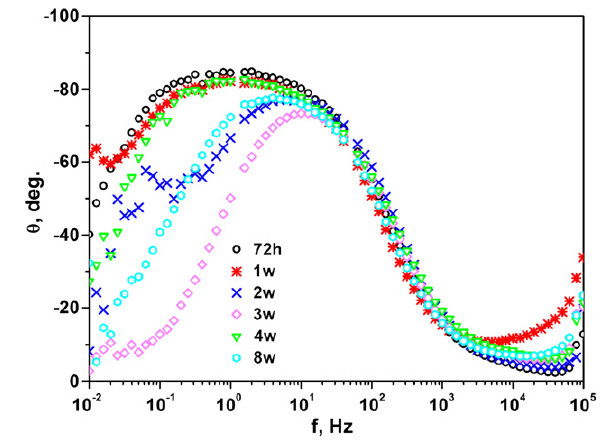
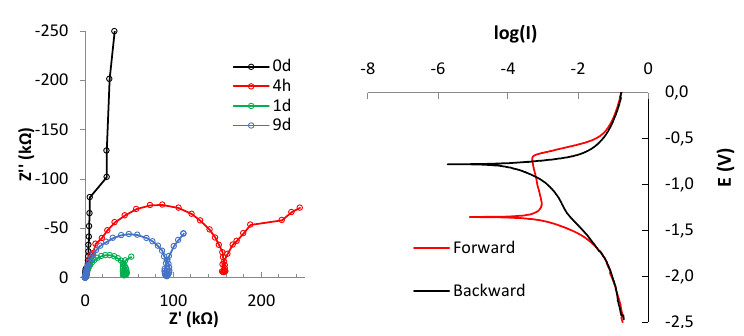
Coated metals
Organic coatings are physical barriers preventing corrosion of metals. Accelerated aging tests as well as electrochemical techniques are implemented to examine the anticorrosion performance of organic coatings. Salt spray tests, potentiodynamic polarization as well as electrochemical impedance spectroscopy have been applied for intact as well as scribed coatings on various metals such as aluminum, steel etc, in combination with microscopy an Raman spectroscopy.