Echem-4-LPMO
Status: Running
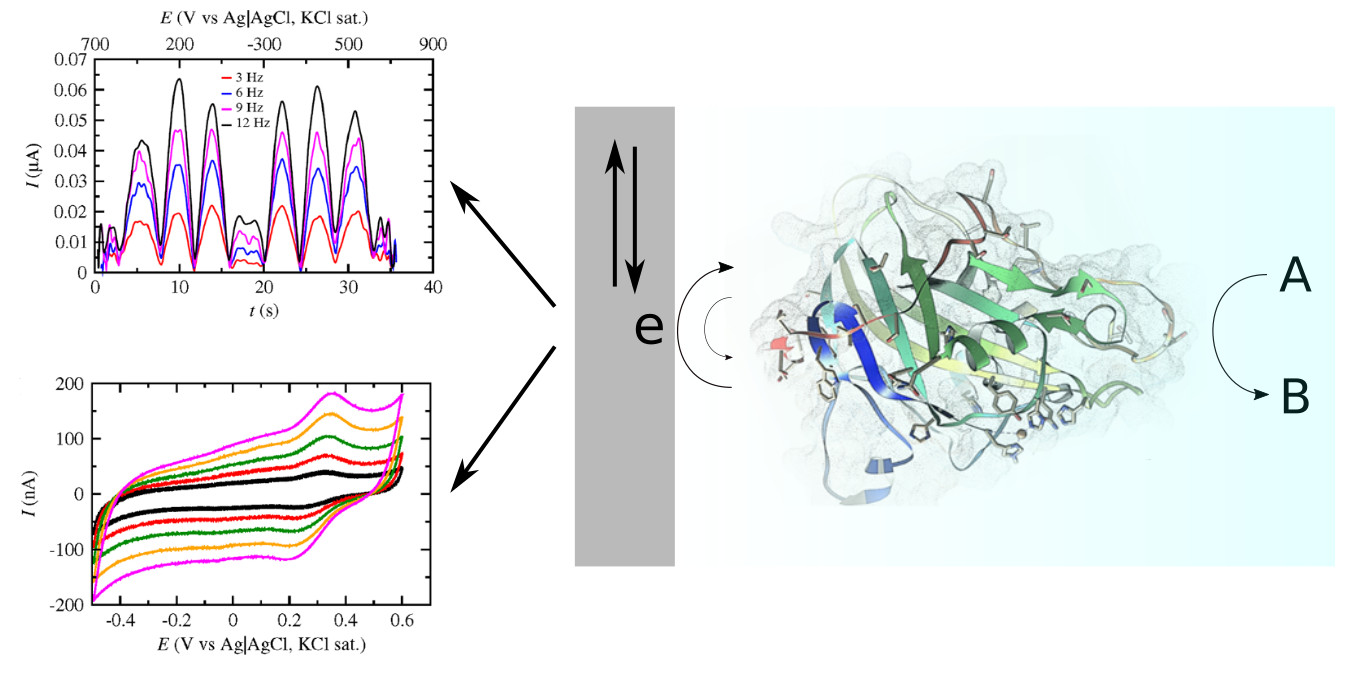
The aim of the research project Echem-4-LPMO is to clarify the mechanism of action of LPMO enzymes that contribute to the enhancement of hydrolytic cleavage of polysaccharides for the production of biofuels, by combining the use of classical and novel electrochemical techniques. Biological pretreatment of the biomass is achieved by the use of cellulotic and hemicellulotic enzymes that are produced by several microorganisms including fungi and bacteria. In most of these organisms apart from the hydrolases, another enzymatic system capable of cleaving glycosidic bonds via an oxidative action, the polysacchiride monoxygenases (PMO), also known as lytic polysacchiride monoxygenases (LPMO) have been found. Both the redox and the catalytic activity of molecules or enzymes can be studied by cyclic voltammetry. This technique offers the great advantage of being applicable to both immobilized (on electrodes) or free (in the solution) species, determining thermodynamic and kinetic quantites. However, in the study of immobilized or free proteins/enzymes with cyclic voltammetry, kinetic analysis is often rendered impossible due to the very low concentration of enzyme and superposition of capacitance currents. For this reason, Fourier Transform Large Amplitude Alternating Current Voltammetry (FTacV) is applied in order to investigate the electron transfer stages of the electrochemical reaction, thus giving more information on the mechanism of the action of the enzymes.
People involved
Publications
A. Karnaouri, K. Chorozian, D. Zouraris, A. Karantonis, E. Topakas, U. Rova, P. Christakopoulos,
"Lytic polysaccharide monooxygenases as powerful tools in enzymatically assisted preparation of nano-scaled cellulose from lignocellulose: A review"
Bioresource Technology, 345 (2022) 126491.D. Zouraris, A. Karnaouri, P.K. Pandis, Chr. Argirusis, E. Topakas and A. Karantonis,
"On the interaction of lytic polysaccharide monooxygenases (LPMOs) with phosphoric acid-swollen cellulose (PASC)"
Journal of Electroanalytical Chemistry, 897 (2021) 115540.D. Zouraris, A. Karnaouri, R. Xydou, E. Topakas and A. Karantonis,
"Exploitation of Enzymes for the Production of Biofuels: Electrochemical Determination of Kinetic Parameters of LPMOs"
Applied Sciences, 11 (2021) 4715.D. Zouraris, A. Karantonis,
"Determination of kinetic and thermodynamic parameters from large amplitude Fourier transform ac voltammetry of immobilized electroactive species"
Journal of Electroanalytical Chemistry, 876 (2020) 114729.
Conferences
K. Chorozian, E. Topakas, A. Karnaouri, D. Zouraris and A. Karantonis,
“A novel multi-step enzymatic process for the isolation of nanocellulose from organosolv pretreated hardwood biomass: insights into the key role of a newly discovered AA9 LPMO” 43rd Symposium on Biomaterials, Fuels and Chemicals (2021).D. Zouraris, A. Karnaouri, P. Pandis, Ch. Argirusis, E. Topakas, A. Karantonis,
“Voltammetric Study of the Binding of Phosphoric Acid-Swollen Cellulose with Immobilized Lytic Polysaccharide Monooxygenases”, XXVI International Symposium on Bioelectrochemistry and Bioenergetics of the Bioelectrochemical Society, Cluj-Napoca, Romania (2021).D. Zouraris, A. Karantonis,
"FTacV for immobilized and free in a solution electroactive species” Graduate Student Symposium on Advantageous Electrochemistry (2020) online edition.
Software
A python3 program for the analysis of FTac voltammograms can be found here